Ethane is chlorinated in a continuous reactor – Ethane chlorination in a continuous reactor is a captivating topic that demands attention in the realm of chemical engineering. This process holds immense significance, as it paves the way for the production of numerous chlorinated hydrocarbons, which serve as vital building blocks for various industries.
In this discourse, we embark on an in-depth exploration of ethane chlorination, unraveling its intricacies and shedding light on its practical applications.
As we delve into the heart of the matter, we will meticulously examine the reactants and products involved in this process, deciphering the intricate dance of chemical transformations. Furthermore, we will dissect the reaction mechanism, unraveling the intricate steps that orchestrate the chlorination of ethane.
Our investigation will extend to the process parameters that govern this reaction, deciphering their profound influence on the efficiency and selectivity of the process.
Introduction to Ethane Chlorination
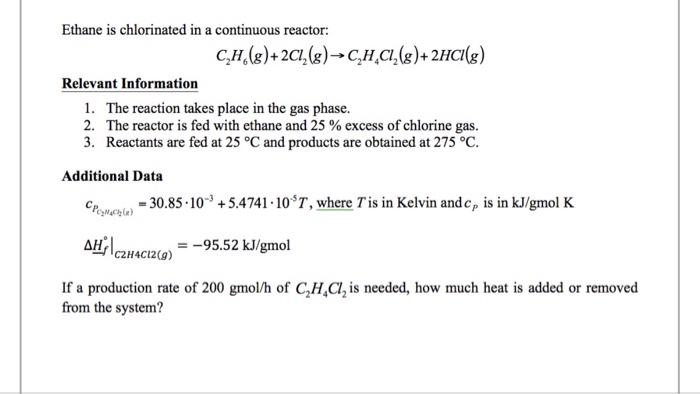
Ethane chlorination is a chemical process that involves the reaction of ethane with chlorine to produce chlorinated hydrocarbons. It is a significant industrial process used in the production of a wide range of products, including vinyl chloride, ethylene dichloride, and polyvinyl chloride (PVC).
The chlorination of ethane is typically carried out in a continuous reactor, which allows for efficient and controlled reaction conditions. In this process, ethane and chlorine are introduced into the reactor, where they react to form chlorinated products.
Reactants and Products
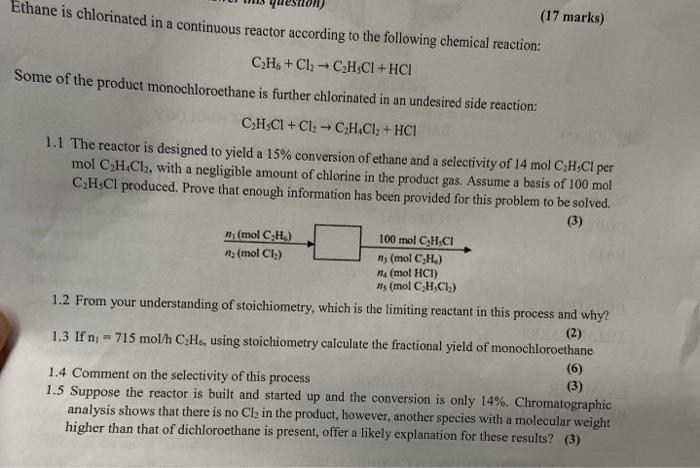
The primary reactants in ethane chlorination are ethane (C2H6) and chlorine (Cl2). Chlorine acts as a reactant, providing the chlorine atoms that are added to the ethane molecule. The main products of the reaction are chlorinated hydrocarbons, including vinyl chloride (C2H3Cl), ethylene dichloride (C2H4Cl2), and polyvinyl chloride (PVC).
As a byproduct of the reaction, hydrochloric acid (HCl) is also formed. This is due to the reaction of hydrogen atoms from the ethane molecule with chlorine atoms.
Reaction Mechanism: Ethane Is Chlorinated In A Continuous Reactor
The chlorination of ethane proceeds through a free radical chain mechanism. This mechanism involves the following steps:
Initiation
The reaction is initiated by the homolytic cleavage of a chlorine molecule, producing two chlorine radicals (Cl •).
“`Cl2 → 2 Cl •“`
Propagation
The chlorine radicals then react with ethane molecules, abstracting a hydrogen atom and forming an ethyl radical (C2H5 •).
“`Cl •+ C2H6 → C2H5 •+ HCl“`
The ethyl radical then reacts with another chlorine molecule, forming an ethyl chloride molecule (C2H5Cl) and a new chlorine radical.
“`C2H5 •+ Cl2 → C2H5Cl + Cl •“`
Termination
The reaction chain is terminated when two radicals react with each other, forming a stable molecule. This can occur through various reactions, such as:
“`C2H5 •+ Cl •→ C2H5Cl“““
C2H5•→ C4H10
“`
Process Parameters

The chlorination of ethane is influenced by several key process parameters, including:
Temperature
Temperature affects the rate of the reaction. Higher temperatures generally lead to faster reaction rates, but they can also promote the formation of unwanted byproducts.
Pressure
Pressure affects the equilibrium of the reaction. Higher pressures favor the formation of chlorinated products, as they shift the equilibrium towards the product side.
Residence Time
Residence time is the amount of time that the reactants spend in the reactor. Longer residence times allow for more complete reactions, but they can also increase the formation of byproducts.
Catalyst
Catalysts can be used to increase the rate of the reaction and improve the selectivity of the process. Common catalysts for ethane chlorination include iron(III) chloride (FeCl3) and aluminum chloride (AlCl3).
Reactor Design

The design of the continuous reactor used in ethane chlorination is crucial for achieving efficient and controlled reaction conditions.
Key considerations in reactor design include:
Reactor Volume
The reactor volume determines the residence time of the reactants in the reactor. A larger reactor volume allows for longer residence times, which can improve the conversion of ethane to chlorinated products.
Residence Time
Residence time is the average time that the reactants spend in the reactor. It affects the extent of the reaction and the selectivity of the process.
Mixing, Ethane is chlorinated in a continuous reactor
Mixing is important to ensure that the reactants are evenly distributed throughout the reactor. Good mixing promotes uniform reaction conditions and prevents the formation of localized hot spots.
Common types of reactors used in ethane chlorination include packed bed reactors and fluidized bed reactors.
Product Separation
After the reaction, the products of ethane chlorination need to be separated from the unreacted reactants and byproducts.
Common methods used for product separation include:
Distillation
Distillation is a process that separates components based on their boiling points. It is used to separate the different chlorinated products from each other and from the unreacted ethane.
Condensation
Condensation is a process that involves cooling a gas or vapor to convert it into a liquid. It is used to condense the chlorinated products from the reaction mixture.
Absorption
Absorption is a process that involves the transfer of a gas or vapor from one phase to another. It is used to remove HCl from the reaction mixture by absorbing it into a solvent.
Environmental Considerations
The chlorination of ethane has several environmental considerations, including:
Formation of Chlorinated Hydrocarbons
The chlorination of ethane produces chlorinated hydrocarbons, which are persistent organic pollutants (POPs). POPs can accumulate in the environment and pose risks to human health and ecosystems.
Waste Disposal
The production of ethane chlorination generates waste products, including HCl and spent catalyst. These waste products need to be disposed of properly to minimize their environmental impact.
Safety Measures
The chlorination of ethane is a hazardous process that requires proper safety measures to protect workers and the environment. These measures include using appropriate personal protective equipment, maintaining proper ventilation, and following established safety protocols.
Popular Questions
What is the primary product of ethane chlorination?
The primary product of ethane chlorination is ethyl chloride, a versatile intermediate used in the production of polyvinyl chloride (PVC) and other chemicals.
What are the environmental concerns associated with ethane chlorination?
Ethane chlorination can release chlorinated hydrocarbons into the environment, which can pose risks to human health and ecosystems. Proper waste management and emission control measures are crucial to mitigate these concerns.