Introducing the Isotopes and Average Atomic Mass Worksheet, an indispensable resource for delving into the fascinating world of isotopes. This worksheet offers a comprehensive exploration of isotopes and their relationship to elements, providing a solid foundation for understanding their significance in various scientific disciplines.
In this guide, we will delve into the fundamental concepts of isotopes, unravel the intricacies of their notation, and explore their impact on average atomic mass. Through engaging exercises and thought-provoking questions, the worksheet fosters a deeper understanding of isotopes and their wide-ranging applications.
Isotopes
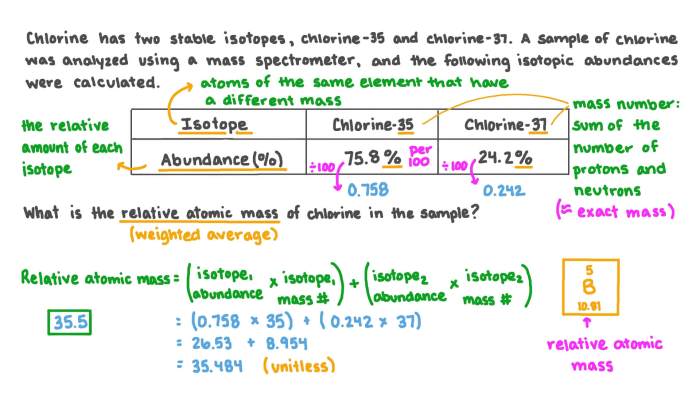
Isotopes are atoms of the same element that have the same atomic number but different mass numbers. The atomic number of an element is the number of protons in its nucleus, which determines the element’s chemical properties. The mass number of an element is the total number of protons and neutrons in its nucleus.
Isotopes are represented by the symbol of the element followed by a superscript indicating the mass number. For example, the isotopes of carbon are 12C, 13C, and 14C. The superscript 12 indicates that 12C has 12 protons and 12 neutrons, while the superscript 13 indicates that 13C has 12 protons and 13 neutrons, and so on.
Average Atomic Mass
The average atomic mass of an element is the weighted average of the masses of all its isotopes, taking into account their relative abundances. The relative abundance of an isotope is the percentage of atoms of that isotope in a naturally occurring sample of the element.
The average atomic mass of an element can be calculated using the following formula:
Average atomic mass = (mass of isotope 1 x abundance of isotope 1) + (mass of isotope 2 x abundance of isotope 2) + … + (mass of isotope n x abundance of isotope n)
For example, the average atomic mass of chlorine is 35.45. This means that a naturally occurring sample of chlorine is composed of 75.77% 35Cl and 24.23% 37Cl.
The average atomic mass of an element is an important property because it is used to calculate the molar mass of the element. The molar mass of an element is the mass of one mole of the element, which is the amount of the element that contains 6.022 x 10 23atoms.
Applications of Isotopes, Isotopes and average atomic mass worksheet
Isotopes have a wide range of applications in medicine, industry, and scientific research. In medicine, isotopes are used for imaging and treating diseases. For example, the isotope 99mTc is used in nuclear medicine to image the heart and other organs.
The isotope 131I is used to treat thyroid cancer.
In industry, isotopes are used to trace chemical reactions and to measure the age of objects. For example, the isotope 14C is used to date archaeological artifacts. The isotope 3H is used to trace the flow of water in aquifers.
In scientific research, isotopes are used to study the structure and function of molecules. For example, the isotope 1H is used to study the structure of water. The isotope 13C is used to study the metabolism of glucose.
FAQ Corner: Isotopes And Average Atomic Mass Worksheet
What is the difference between isotopes and elements?
Isotopes are variations of the same element, differing in the number of neutrons in their nuclei, while elements are distinct substances with unique atomic numbers.
How is average atomic mass calculated?
Average atomic mass is calculated by multiplying the mass of each isotope by its relative abundance and summing the products.
What are the applications of isotopes?
Isotopes have a wide range of applications, including in medicine (e.g., medical imaging, cancer treatment), industry (e.g., material testing, tracing chemical reactions), and scientific research (e.g., dating objects, studying geological processes).