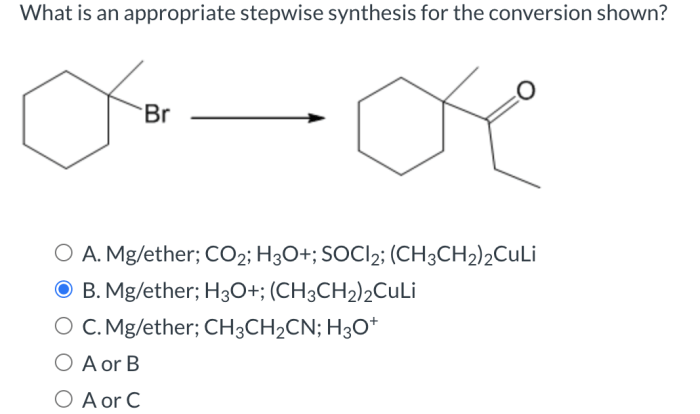
Name the aldehyde displayed below. – Name the aldehyde displayed below: A Comprehensive Guide delves into the fascinating world of aldehydes, exploring their structure, identification, chemical properties, and diverse applications. Embark on a journey of discovery as we unveil the secrets of these versatile organic compounds.
Aldehydes, characterized by their distinctive carbonyl group, play a pivotal role in various industries, from chemical synthesis to food production. Their unique reactivity makes them indispensable building blocks for countless products that enhance our daily lives.
Definition of Aldehydes
Aldehydes are organic compounds characterized by the presence of a carbonyl group (C=O) bonded to at least one hydrogen atom and other carbon or hydrogen atoms. The carbonyl group is the functional group that defines aldehydes and is responsible for their chemical properties.In
the IUPAC nomenclature system, aldehydes are named by adding the suffix “-al” to the parent alkane name. The parent alkane name indicates the number of carbon atoms in the aldehyde’s carbon chain. For example, the aldehyde with the formula CH3CHO is named methanal, as it contains one carbon atom and the aldehyde functional group.
IUPAC Nomenclature of Aldehydes
The IUPAC nomenclature system for aldehydes follows specific rules:
- Identify the parent chain: The parent chain is the longest continuous chain of carbon atoms that contains the aldehyde group.
- Number the parent chain: The parent chain is numbered from the carbon atom of the aldehyde group to give it the lowest possible number.
- Identify the substituents: Any atoms or groups of atoms attached to the parent chain are called substituents.
- Name the substituents: Substituents are named according to the IUPAC rules for naming organic compounds.
- Combine the name of the parent chain, substituents, and suffix: The name of the aldehyde is formed by combining the name of the parent chain, the names of the substituents, and the suffix “-al”.
Structure of the Given Aldehyde
The given aldehyde has the chemical formula CH3CH2CHO. It is a three-carbon aldehyde with the IUPAC name propanal.
Chemical Structure
The chemical structure of propanal can be represented as follows:
 |
Propanal CH3CH2CHO |
The key functional group in propanal is the aldehyde group (-CHO). The aldehyde group consists of a carbon atom double-bonded to an oxygen atom and single-bonded to a hydrogen atom. The aldehyde group is the reactive site of the molecule and is responsible for the characteristic reactions of aldehydes.Other
structural features of propanal include the two methyl groups (-CH3) and the methylene group (-CH2-). The methyl groups are located at the ends of the carbon chain, while the methylene group is located in the middle of the carbon chain.
Identification of the Aldehyde
Identifying aldehydes is crucial for various scientific disciplines, including organic chemistry and biochemistry. Several methods are employed to identify aldehydes, each with its own advantages and limitations.
Spectroscopic Techniques
Spectroscopic techniques provide valuable information about the functional groups present in a molecule. Two commonly used spectroscopic techniques for aldehyde identification are:
-
-*Infrared (IR) Spectroscopy
Aldehydes exhibit a characteristic strong absorption band in the range of 1700-1750 cm -1, corresponding to the C=O stretching vibration.
-*Nuclear Magnetic Resonance (NMR) Spectroscopy
Aldehyde protons typically resonate between 9-10 ppm in the 1H NMR spectrum. The adjacent methylene protons also show characteristic splitting patterns.
Chemical Reactions
Chemical reactions can also be used to identify aldehydes. Some common reactions include:
-
-*2,4-Dinitrophenylhydrazine (2,4-DNP) Test
Aldehydes react with 2,4-DNP to form a yellow-orange precipitate.
-*Tollens’ Test
Aldehydes reduce silver ions (Ag +) to metallic silver, forming a silver mirror on the inner surface of the test tube.
-*Schiff’s Test
Aldehydes react with Schiff’s reagent (fuchsin-sulfurous acid) to form a purple or magenta color.
Chemical Properties of Aldehydes
Aldehydes are highly reactive compounds due to the presence of the carbonyl group. They undergo a variety of chemical reactions, including oxidation, reduction, and nucleophilic addition.
Oxidation
Aldehydes are easily oxidized to carboxylic acids. This reaction is typically carried out using oxidizing agents such as potassium permanganate (KMnO4) or potassium dichromate (K2Cr2O7). The oxidation of aldehydes is a common method for the preparation of carboxylic acids.
KMnO4 + 3RCHO → 3RCOOH + MnO2 + H2O
Reduction
Aldehydes can be reduced to primary alcohols using reducing agents such as sodium borohydride (NaBH4) or lithium aluminum hydride (LiAlH4). The reduction of aldehydes is a common method for the preparation of primary alcohols.
NaBH4 + 4RCHO → 4RCH2OH + NaOH
Nucleophilic Addition
Aldehydes undergo nucleophilic addition reactions with a variety of nucleophiles. Nucleophiles are species that have a lone pair of electrons that can be donated to the carbonyl group. The nucleophilic addition of water to aldehydes forms hemiacetals, which can further react to form acetals.
RCHO + H2O → RCH(OH)2RCH(OH)2 + RCHO → RCH(OR)2 + H2O
The nucleophilic addition of alcohols to aldehydes forms acetals. Acetals are stable compounds that can be used to protect the carbonyl group from further reactions.
RCHO + 2R’OH → RCH(OR’)2 + H2O
The nucleophilic addition of amines to aldehydes forms imines. Imines are important intermediates in the synthesis of many heterocyclic compounds.
RCHO + R’NH2 → RCH=NR’ + H2O
Applications of Aldehydes: Name The Aldehyde Displayed Below.
Aldehydes are highly versatile compounds with a wide range of applications across various industries. Their unique chemical properties, particularly their reactivity towards nucleophiles, make them valuable starting materials for chemical synthesis, food production, and pharmaceuticals.
Chemical Synthesis
In the chemical industry, aldehydes serve as building blocks for the synthesis of more complex organic compounds. They are commonly used in the production of polymers, plastics, and synthetic fragrances. For instance, formaldehyde is a key component in the production of polyoxymethylene (POM), a high-performance plastic used in automotive parts, appliances, and medical devices.
Food Production, Name the aldehyde displayed below.
Aldehydes play a significant role in the food industry, contributing to the flavor and aroma of various food products. Vanillin, derived from vanillin, is a widely used flavoring agent in desserts, beverages, and confectionery. Additionally, cinnamaldehyde, found in cinnamon, is responsible for the characteristic flavor and aroma of cinnamon-flavored products.
Pharmaceuticals
Aldehydes are essential intermediates in the synthesis of numerous pharmaceuticals. For example, benzaldehyde is a precursor to the production of benzylpenicillin, a widely used antibiotic. Salicylaldehyde, derived from salicylic acid, is a key ingredient in the production of aspirin, a common pain reliever and anti-inflammatory drug.
FAQ Insights
What is the IUPAC nomenclature for aldehydes?
Aldehydes are named using the suffix “-al” added to the root name of the parent alkane.
How can aldehydes be identified?
Aldehydes can be identified using various methods, including spectroscopic techniques (e.g., NMR, IR) and chemical reactions (e.g., Tollens’ test, Fehling’s test).
What are the characteristic chemical reactions of aldehydes?
Aldehydes undergo a range of reactions, including oxidation, reduction, and nucleophilic addition. These reactions are fundamental to their applications in organic synthesis.